Buy Now
Biological Safety Cabinet Market Size, Share, Growth & Industry Analysis, By Type (Class I, Class II, Class III), By End User (Pharmaceutical and Biotechnology Companies, Academic and Research Laboratories, Diagnostics Laboratories), and Regional Analysis, 2024-2031
Pages: 170 | Base Year: 2023 | Release: February 2025 | Author: Sunanda G.
A biological safety cabinet (BSC) is a ventilated, enclosed laboratory workspace designed to safely handle biohazardous materials by protecting personnel, the environment, and the samples. It utilizes controlled airflow and high-efficiency particulate air (HEPA) filtration, which prevents contamination.
Widely used in microbiology, biotechnology, and pharmaceutical research, BSCs are essential for maintaining biosafety standards in laboratories working with infectious or hazardous biological materials.
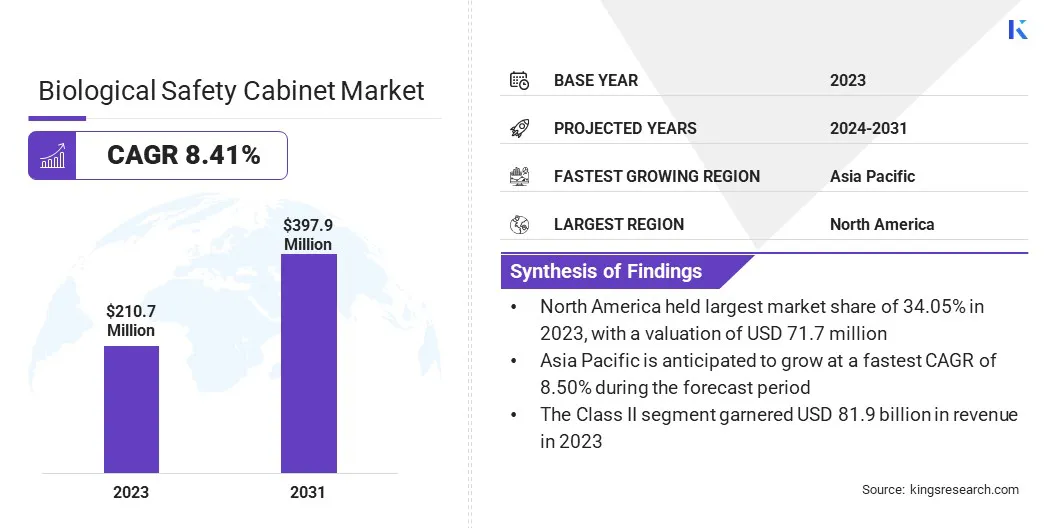
The global biological safety cabinet market size was valued at USD 210.7 million in 2023, which is estimated to be valued at USD 226.0 million in 2024 and reach USD 397.9 million by 2031, growing at a CAGR of 8.41% from 2024 to 2031.
The growth of the market is driven by increasing demand for laboratory safety in the research and healthcare sectors, particularly in the biotechnology and pharmaceutical industries.
Strict government regulations ensuring worker protection and contamination control further boost market expansion. Additionally, innovations in product design, such as enhanced user workspace and integrated safety features, are enhancing the appeal of BSCs.
Major companies operating in the biological safety cabinet industry are Thermo Fisher Scientific Inc., Kewaunee International Group, The Baker Company, Labconco, FASTER S.r.l., Syntegon Telstar, SLU, Air Science USA, Haier Biomedical, Cruma S.A., Berner International GmbH, Germfree, Esco Micro Pte. Ltd., Biolab Scientific, LaboGene, LAMSYSTEMS CC, and others.
Increasing financial support for life sciences and biotechnology research is contributing to the growth of the market. Pharmaceutical companies, research institutions, and government agencies are allocating substantial budgets to drug discovery, vaccine development, and microbiological studies.
Expanding R&D activities in molecular biology and cell culture research is fueling the demand for biosafety cabinets to maintain contamination-free environments. Investments in biotechnology startups and academic research facilities are further increasing the adoption of advanced laboratory equipment.
Market Driver
"Growing Focus on Laboratory Safety and Worker Protection"
Rising awareness of occupational safety in research and healthcare settings is propelling the growth of the biological safety cabinet market. Laboratory-acquired infections and exposure to hazardous biological agents pose significant health risks to researchers and technicians.
Institutions are prioritizing investments in biosafety cabinets to comply with workplace safety regulations and minimize contamination risks. The adoption of protective laboratory equipment in hospitals, pharmaceutical companies, and academic research centers is growing due to increased safety concerns.
The integration of these high-performance systems highlights the growing demand for biological safety cabinets, particularly in the biotechnology and pharmaceutical research sectors.
Expanding initiatives to improve laboratory protocols and training programs on biosafety measures are further highlighting the need for high-performance biosafety cabinets across various industries.
Market Challenge
"Supply Chain Disruptions"
A key challenge impeding the growth of the biological safety cabinet market is supply chain disruptions, which hinder the timely procurement of essential components. Global shortages in materials, including specialized filters and electronics, are resulting in production delays.
To mitigate this challenge, companies are diversifying their supplier base, strengthening local partnerships, and utilizing advanced manufacturing technologies to reduce dependence on external sources.
Additionally, strategic inventory management practices, such as just-in-time systems, are being employed to optimize product availability and minimize delays in delivering critical laboratory equipment.
Market Trend
"Expansion of Biopharmaceutical and Vaccine Development"
The biological safety cabinet market is expanding due to increasing R&D activities in biopharmaceuticals and vaccine production. Rising demand for monoclonal antibodies, gene therapies, and cell-based treatments is increasing investments in laboratory infrastructure.
Vaccine manufacturers are deploying biosafety cabinets to ensure aseptic conditions during formulation and quality control processes. Biopharmaceutical firms involved in recombinant DNA technology and protein synthesis are integrating advanced containment solutions to maintain biosafety standards.
Growth in global immunization programs and strategic collaborations in vaccine research are further reflecting the need for high-efficiency biological safety cabinets in pharmaceutical manufacturing and clinical testing environments.
|
Segmentation |
Details |
|
By Type |
Class I, Class II, Class III |
|
By End User |
Pharmaceutical and Biotechnology Companies, Academic and Research Laboratories, Diagnostics Laboratories |
|
By Region |
North America: U.S., Canada, Mexico |
|
Europe: France, U.K., Spain, Germany, Italy, Russia, Rest of Europe |
|
|
Asia-Pacific: China, Japan, India, Australia, ASEAN, South Korea, Rest of Asia-Pacific |
|
|
Middle East & Africa: Turkey, UAE, Saudi Arabia, South Africa, Rest of Middle East & Africa |
|
|
South America: Brazil, Argentina, Rest of South America |
Market Segmentation:
Based on region, the global market has been classified into North America, Europe, Asia Pacific, Middle East & Africa, and Latin America.
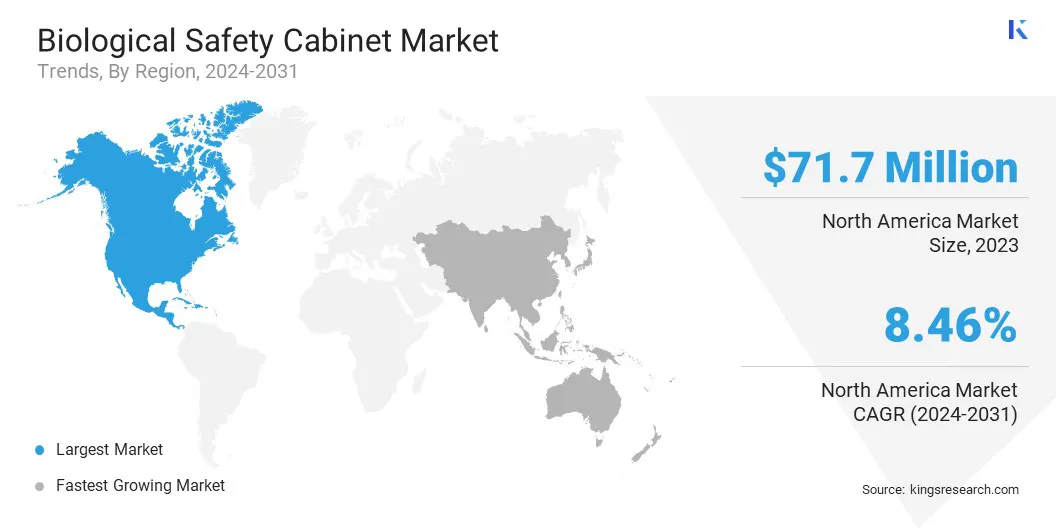
The North America biological safety cabinet market captured a substantial share of around 34.05% in 2023, valued at USD 71.7 million. North America, particularly the United States, is witnessing increased investments in healthcare infrastructure, including the expansion of biotechnology and pharmaceutical research facilities.
These investments support the need for high-quality lab equipment, including biological safety cabinets, to maintain safety standards in laboratories and healthcare environments, aiding regional market growth.
Additionally, a surge in biotechnology investments, particularly within research and development, is boosting this expansion. As companies focus on innovations in genomics, cell biology, and personalized medicine, significant funding is being allocated to advanced research labs. This investment is expected to fuel sustained market growth in the region.
Asia Pacific biological safety cabinet industry is set to grow at a CAGR of 8.50% over the forecast period. The region is witnessing a surge in biotech startups and expanding life sciences research initiatives.
With the rise in venture capital funding and collaborations, there is an increasing demand for high-quality laboratory equipment, including biological safety cabinets, to ensure the safe handling of biological substances. This trend is particularly evident in China, South Korea, and India, where government policies and global partnerships are promoting growth in biotech and pharmaceuticals
The biological safety cabinets industry is characterized by a large number of participants, including both established corporations and emerging players. Several market participants are incorporating advanced technologies such as HEPA filtration systems, UV sterilization, and touchless controls to improve the cabinet's performance, ensuring higher protection against biological contaminants.
In addition, the designs are being optimized to offer a more efficient workspace, with increased internal volumes and ergonomic features that allow better movement and functionality for users. These innovations are boosting the growth of the BSC market by meeting the evolving needs of research, healthcare, and biotech industries.
Recent Developments (M&A/New Product Launch)
Frequently Asked Questions